By Julia A. Rucker, MSW/MPH, Molly T. Beinfeld, MPH, and James D. Chambers, PhD, MPharm, MSc
October 2, 2023
Introduction
In this blog we consider the impact of CVS Caremark’s 2019 acquisition of Aetna on Aetna’s enrollees’ access to specialty drugs. We find that, following the acquisition, Aetna had adjusted coverage criteria in the majority of their specialty drug coverage policies. In most cases specialty drug coverage became more generous, with Aetna either removing coverage restrictions or making existing coverage restrictions less onerous.
Health plans guide their enrollees’ access to specialty drugs through medical or pharmacy coverage policies. Research has found that plans’ specialty drug coverage policies can vary, meaning that patients enrolled in different health plans may have different access to the same drugs. On occasion, as in the case of CVS-Aetna, two health plans merge to form a single larger plan.
Methods
Data Source
We used the Tufts Medical Center Specialty Drug Evidence and Coverage Database (SPEC), which includes specialty drug coverage decisions issued by 17 large US commercial health plans. We compared coverage policies issued by CVS and Aetna for the same set of specialty drugs that were current before (August 2018) and after (August 2020) CVS Caremark’s acquisition of Aetna. We compared coverage policies using the following patient access criteria:
- Patient subgroup restrictions – Requirement for patients to meet particular clinical criteria (e.g., experiencing symptoms of particular severity or duration);
- Step therapy protocols – Requirement for patients to first try and fail an alternative drug or treatment;
- Prescriber requirements – Requirement for a certain type of physician to prescribe a drug;
- Any other restriction type – For example, a requirement for a drug to be used in combination with another treatment
Sample and Analyses
Our sample included 188 drug-indication pairs (i.e., infliximab for rheumatoid arthritis) for which both Aetna and CVS had issued a coverage policy at both time points. We examined the following:
- The concordance between CVS and Aetna’s coverage policies at each time point;
- The proportion of Aetna’s coverage policies that changed between the first and second time points, i.e., (i) became more restrictive (i.e., added a coverage requirement), (ii) became less restrictive (i.e., removal or relaxation of a coverage requirement), or (iii) remained the same;
Results
For the 188 drug-indication pairs, 14% of Aetna’s and CVS’s specialty drug coverage policies were concordant pre-merger and 82% were concordant post-merger (Figure 1).
Aetna’s coverage policies became more generous 42% of the time, 12% became more restrictive and 46% remained unchanged after the merger (Figure 2).
Of the 23 (12%) Aetna coverage policies that became more restrictive, the increase in restrictiveness was most often due to adjustments in patient subgroup restrictions (56% of changes) and step therapy protocols (35%). For example, for abatacept (Orencia®) for rheumatoid arthritis, Aetna did not impose step therapy requirements in 2018, but in 2020 required that patients ‘step through’ both methotrexate and a biologic before being eligible for treatment.
Conclusion
Our findings suggest that the CVS-Aetna merger affected Aetna enrollees’ access to specialty drugs. Most often, specialty drug coverage became more generous, although coverage became more restrictive 12% of the time.
More on the Specialty Drug Evidence and Coverage (SPEC) Database
The Specialty Drug Evidence and Coverage (SPEC) Database is a first-of-its-kind resource designed to enhance the transparency of commercial health plan specialty drug coverage. SPEC draws on publicly available medical and pharmacy coverage policies issued by 18 of the largest commercial health plans. Health plans in SPEC cover roughly 170 million lives, or 70% of the market. SPEC includes 399 drugs manufactured by 105 different pharmaceutical and biotech companies. We keep SPEC timely by updating its contents every four months. SPEC includes information on three principal components: (1) health plan coverage decisions, (2) the evidence health plans cite in their coverage policies, and (3) specialty drug attributes.
Access to SPEC is through the CEVR Sponsorship program. Access to the online search portal of the SPEC database is also available for individuals from academic organizations for non-commercial use of the data. Contact James Chambers (jchambers@tuftsmedicalcenter.org) for more information.
Figure 1. Concordance of CVS and Aetna specialty drug coverage policies pre and post-merger (n= 188 drug/indication pairs)
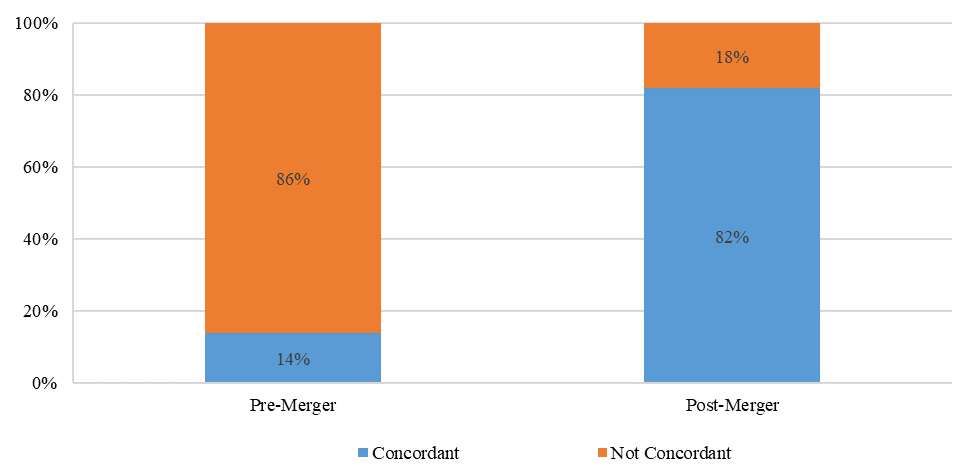
Notes: Concordant means that CVS and Aetna covered a particular drug-indication pair for the same patient population
Figure 2. Changes in Aetna specialty drug coverage policies post-merger (n=188 drug-indication pairs)
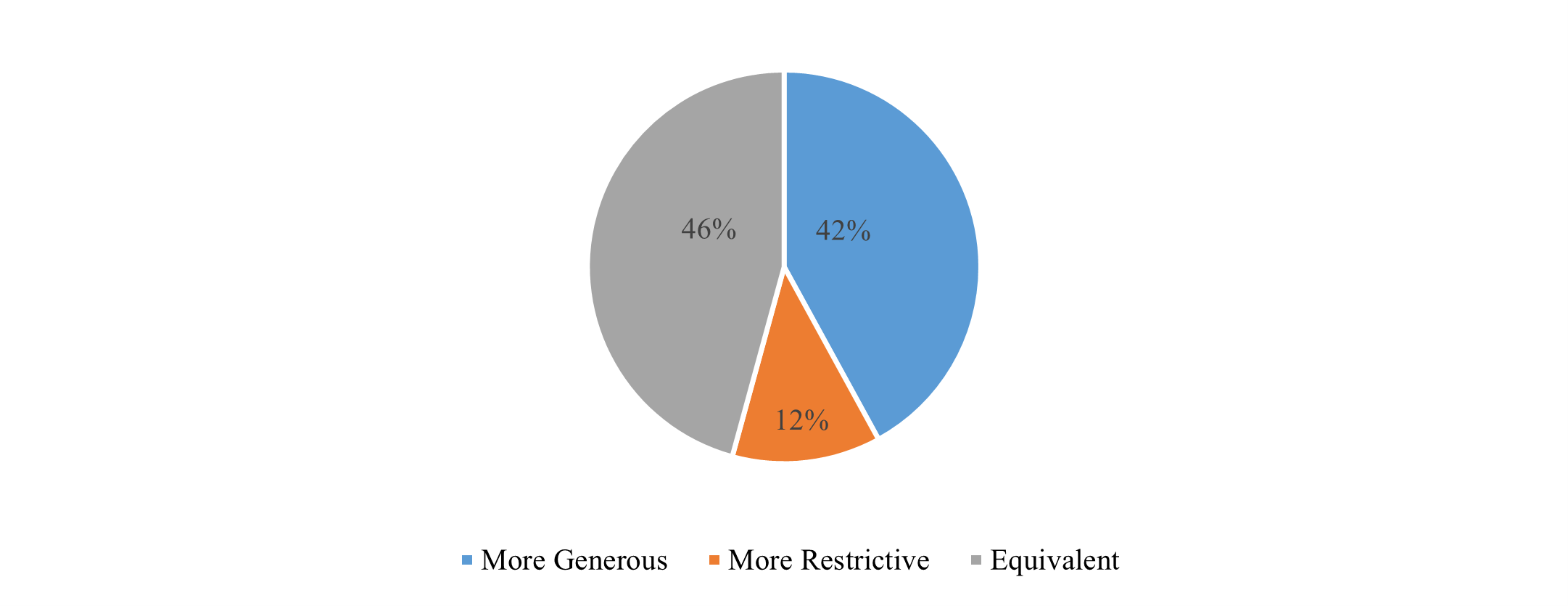
Table 1. Drugs with more restrictive coverage for Aetna enrollees post-merger, August 2020