By Daniel Enright, MS, Molly T. Beinfeld, MPH, and James D. Chambers, PhD, MPharm, MSc
August 17, 2023
Introduction
Two years after its approval, questions remain about how US health plans cover Wegovy® (semaglutide) for obesity management.1,2 High annual costs and high demand for semaglutide have left payers struggling with how to cover the drug while minding budget consequences. We identified the requirements US commercial health plans are implementing in their semaglutide coverage policies and found they are restricting access to the drug, but coverage requirements vary considerably.
Methods & data sources
We reviewed coverage policies for semaglutide issued by large US commercial health plans, focusing on the following requirements:
- Weight restrictions (e.g., BMI > 30 kg/m2)
- Pharmacological step therapy requirements
- Diet and exercise requirements
- Requirement for patients to enroll in a behavioral modification program prior to gaining access
- Approval duration
- Continuation criteria
Our data come from the Tufts-CEVR Specialty Drug Evidence and Coverage (SPEC) database, which contains detailed information on how 18 of the largest US commercial health plans cover specialty drugs and products. Coverage was current as of April 2023.
How do plans cover semaglutide for obesity?
Of the 18 health plans examined, 7 did not issue a policy for semaglutide. The 11 plans that issued a policy varied in their coverage requirements.
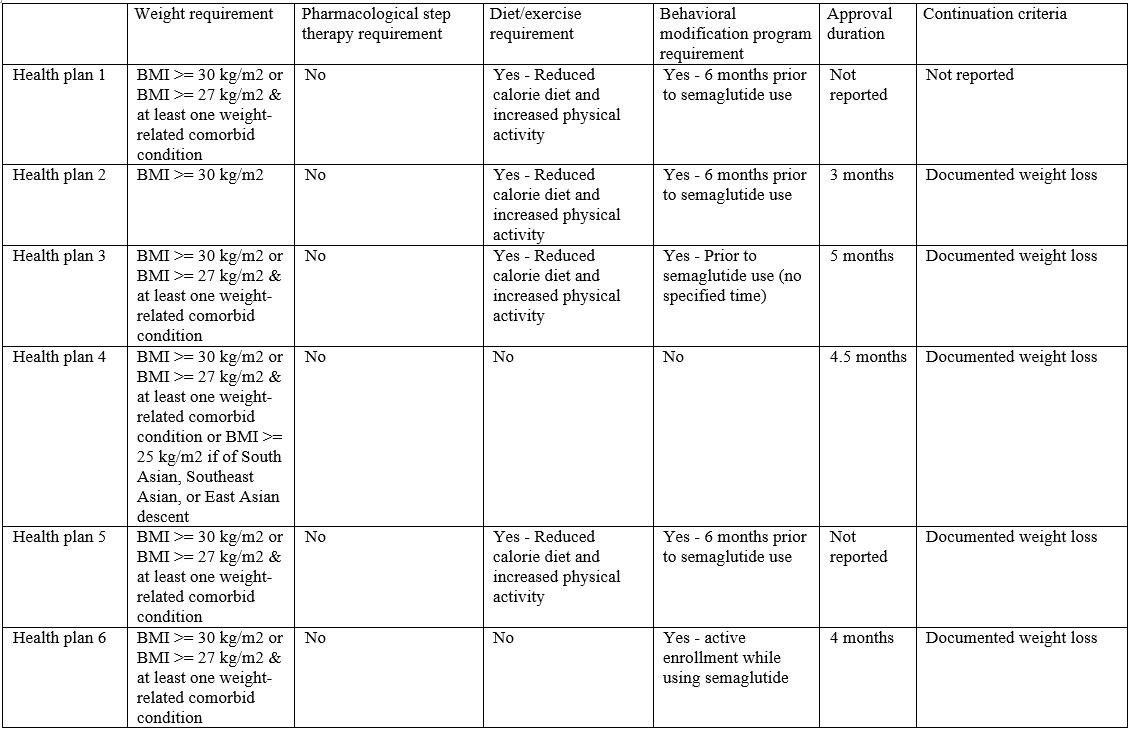
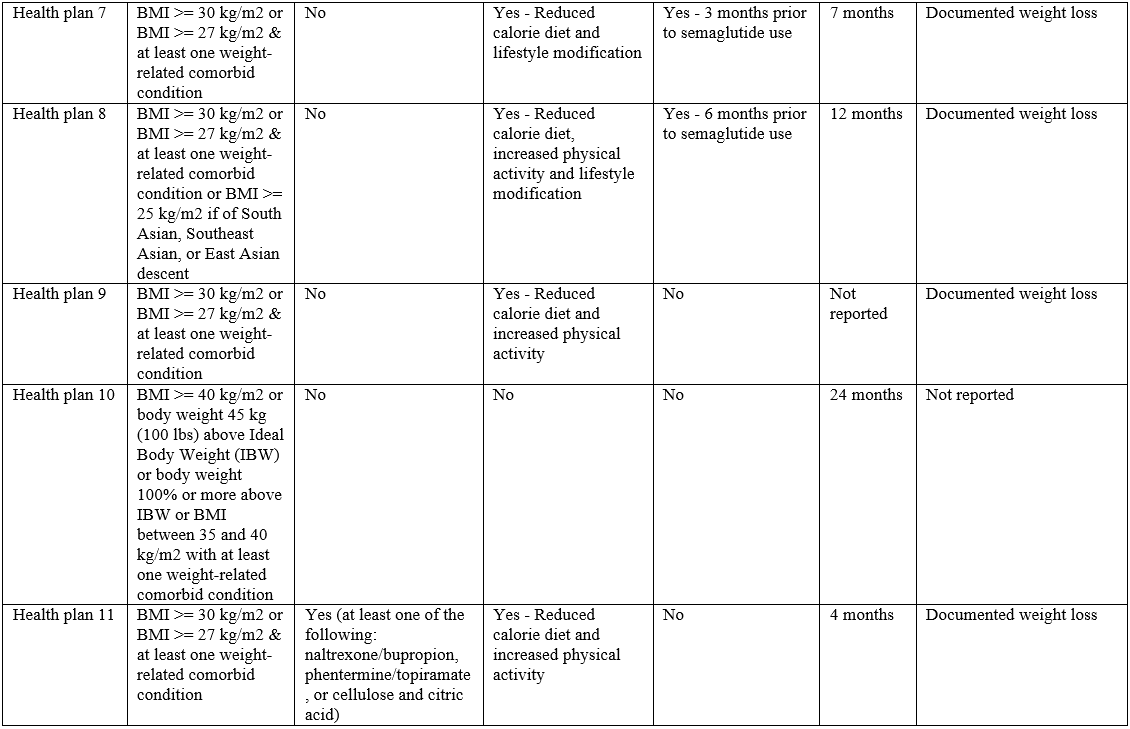
Weight requirements
All 11 health plans covering semaglutide specified a BMI or other weight restriction (Table 1). Nine imposed BMI requirements in-line with semaglutide’s labeled indication (i.e., BMI > 30 kg/m2 or BMI > 27 kg/m2 & at least one weight-related comorbid condition). Two imposed more restrictive weight requirements than the label: one only covering individuals with a BMI > 30 kg/m2, and the other implementing BMI requirements exceeding 30 kg/m2.
Pharmacological step therapy requirements
One plan implemented a pharmacological step therapy (Table 1), requiring patients to try at least one treatment (i.e., naltrexone/bupropion, phentermine/topiramate, or cellulose and citric acid) before authorizing coverage for semaglutide.
Diet and exercise requirements
Eight health plans required patients to use semaglutide in addition to a reduced calorie diet, increased physical activity and/or other lifestyle modifications.
Behavioral modification program requirements
Seven health plans required patients to enroll in a behavioral modification program in order to receive semaglutide; 6 plans require enrollment prior to initiating the drug, while one plan required enrollment as an adjunct to therapy.
Recent update in FDA label indication
In December 2022, FDA expanded semaglutide’s label to include “pediatric patients aged 12 years and older with an initial BMI at the 95th percentile or greater for age and sex”.3 At the time of analysis (April 2023), only 4 health plans had updated their coverage policy to include this additional approved use. In other words, 7 plans limited coverage to adult patients only; however, it is likely that these plans will broaden coverage to include patients 12 years and older when they next update their policies.
Approval duration and reauthorization requirements
Eight health plans reported an approval duration for semaglutide. While approval duration was generally short, it varied substantially among plans (12 weeks to 2 years). All of the 9 health plans that reported continuation criteria required patients to have documented weight loss to continue therapy. All health plans required weight loss of at least 4% or 5% from baseline, with 2 plans also requiring patients have a documented BMI no greater than 25 kg/m2.
Discussion
The largest US commercial health plans vary in how they cover semaglutide for obesity. Variation in coverage can lead to unequal access, as coverage often depends on which health plan an employer offers. With high obesity rates in the US,4 millions of commercially covered patients may face barriers to accessing semaglutide.
Our analysis also found a lack of transparency about how health plans cover semaglutide, as 7 of the 18 included plans did not issue a public coverage policy for the drug. Non-issuance of a coverage policy does not necessarily mean non-coverage, though it does mean that coverage information is not publically available.
We also found that among health plans that issue coverage policies, weight thresholds, diet/exercise requirements and step therapy protocols were largely in line with the FDA label. Enrollment in a formal behavioral modification program was a common requirement for access to semaglutide, which could create barriers to access, as there is ambiguity around what formal enrollment means and who will pay for these programs as well as a lack of data to support the use of these programs for weight loss.
This analysis only examines coverage policies for semaglutide, not other potential barriers such as co-pays and coinsurance. Further, some employers may exclude semaglutide or any weight loss treatments from their formularies, regardless of publicly-available coverage policies.2,5 Equity issues also exist when it comes to access of semaglutide, as obesity disproportionally affects Black and Hispanic US adults.4
According to a survey of payers, data on clinical outcomes beyond weight loss, such as cardiovascular outcomes, may be needed before they cover the drug.2 Top-line results from the SELECT cardiovascular outcomes trial showed reduction in risk of stroke, myocardial infarction, or cardiovascular death with semaglutide in patients with established cardiovascular disease.6 It will be important to see how payers respond to this information, and the availability of additional options, such as tirzepatide, expected to be approved this year.
References
- Wainer D. Demand for obesity drugs has insurers paying close attention. Wall Street Journal. April 17, 2023. https://www.wsj.com/articles/demand-for-obesity-drugs-has-insurers-paying-close-attention-7330d093
- Pharmaceutical Strategies Group (PSG). Trends in drug benefit design report. 2023. https://rxss.com/benefit-design-report-2023/
- Novo Nordisk. Wegovy® (semaglutide) [package insert]. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215256s005lbl.pdf
- Centers for Disease Control and Prevention. Adult obesity facts. https://www.cdc.gov/obesity/adult-obesity-facts/index.html
- Matthews AW. Your company doesn’t want you to take Ozempic for weight loss. Here’s why. Wall Street Journal. May 22, 2023. https://www.wsj.com/articles/workers-want-ozempic-for-weight-loss-few-employers-want-to-pay-for-it-215fb5c1
- Novo Nordisk. Semaglutide 2.4 mg reduces the risk of major adverse cardiovascular events by 20% in adults with overweight or obesity in the SELECT trial. August 8, 2023. https://www.novonordisk.com/news-and-media/news-and-ir-materials/news-details.html?id=166301
Table 1. US commercial health plan coverage of semaglutide for obesity, April 2023