By Katherine A. Clifford, MPH, Molly T. Beinfeld, MPH, and James D. Chambers, PhD, MPharm, MSc
December 13, 2023
December 8th, 2023 was a landmark day for Sickle Cell disease (SCD) patients, as the US Food and Drug Administration (FDA) approved two gene therapies for the disease. These therapies represent important advancements for a disease with a high unmet clinical need but come with eye-watering prices - $2.2m for exagamglogene autotemcel (exa-cel) and $3.1m for lovotibeglogene autotemcel (lovo-cel).1 Questions regarding the duration and efficacy in real-world populations introduce further financial uncertainties to payers. 2
To gain insight into how payers may cover these therapies, in this blog we examine how the largest US commercial payers cover existing SCD treatments.
How might health plans cover the gene therapies for SCD?
Sickle Cell disease refers to a grouping of inheritable red blood cell disorders in which hemoglobin is structurally abnormal. Patients with SCD suffer from vaso-occlusive crises (VOCs). VOCs occur when sickled cells become stuck in small blood vessels and, blocking blood flow and causing severe pain, often leading to hospitalization.3
Management is costly and includes intravenous fluids, pain-reducing medications, and hospitalization.4 In addition to hydroxyurea, long considered the standard of care, two treatments to reduce the frequency of VOCs include: 1) crizanlizumab-tmca, and 2) voxelotor.5
For exa-cel, 94.1% of participants in clinical trials remained VOC-free for greater than 12 consecutive months. Similarly, for lovo-cel 90% of participants achieved complete resolution of VOCs between six and 18 months of lovo-cel infusion.6
Understanding how US payers cover existing SCD treatments provides insight into how they may cover the new gene therapies.
We examined: 1) payer coverage of crizanlizumab-tmca and voxelotor, and 2) payer coverage of existing gene therapies.
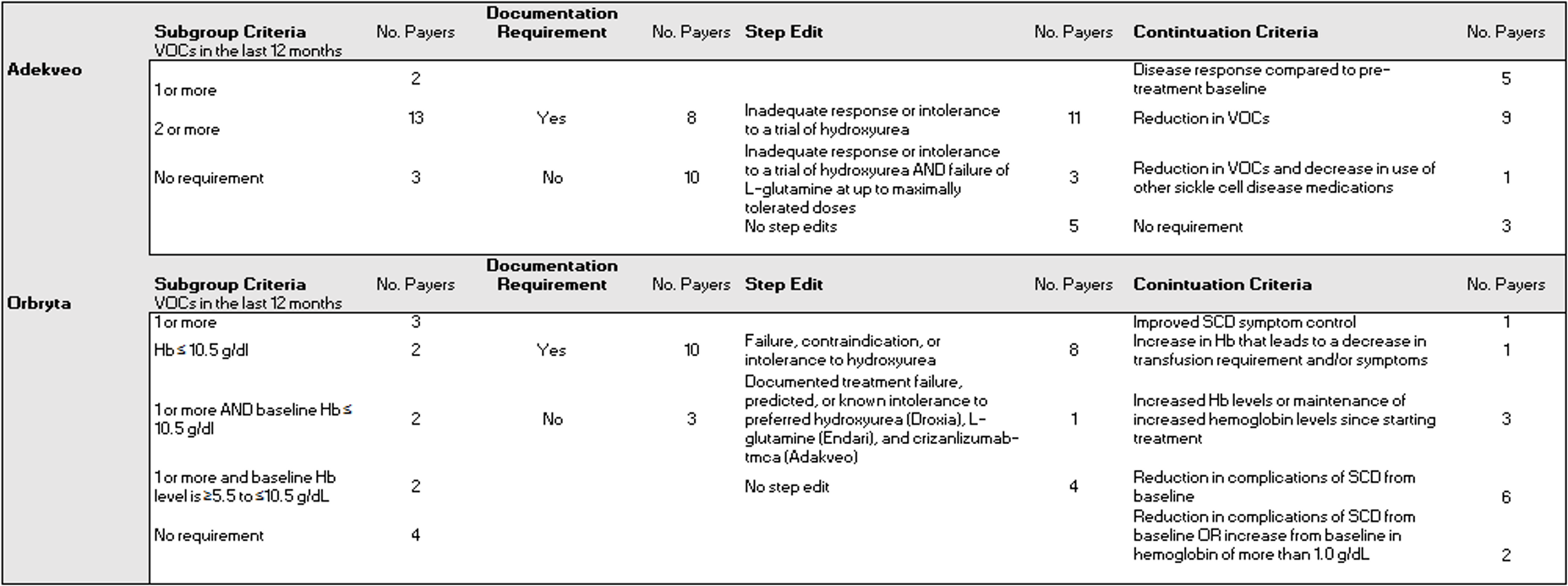
Our data come from the Tufts-CEVR Specialty Drug Evidence and Coverage (SPEC) Database, which contains detailed information on how 18 of the largest US commercial health plans cover specialty drugs and products. As of August 2023, SPEC included 18 coverage policies for crizanlizumab and 13 for voxelotor. Notably, all policies – for both therapies - included restrictions that limit access beyond the FDA labeled indication.
Subgroup restrictions
For crizanlizumab, fifteen payers included subgroup restrictions related to disease severity, although details varied across payers. Payers most often required that patients have experienced between 2 and 10 VOCs in the past year (Table 1), a requirement consistent with the inclusion criteria from the drug’s registration study.7
For voxelotor, nine payers included subgroup restrictions related to disease severity, specifically with respect to VOC frequency and hemoglobin level. These requirements varied across payers (Table 1) but were generally consistent with the drug’s registration study inclusion criteria of at least one VOC and hemoglobin ≥5.5 to ≤10.5 g/dL during screening.8
Step Therapy
Fourteen payers imposed step therapy requirements on crizanlizumab, including 11 payers that required patients to first try hydroxyurea, and three payers that required for patients to first try with hydroxyurea AND L-glutamine (Table 1).
For voxelotor, 8 payers required that patients first try hydroxyurea. One payer required that patients first try three preferred agents 1) hydroxyurea 2) L-glutamine and 3) Crizanlizumab-tmca (Table 1).
Continuation Criteria
Fifteen payers explicitly required that patients had benefited from crizanlizumab before granting reauthorization. How these payers defined clinical benefit varied, from requiring an undefined “disease response” compared to pre-treatment baseline (5 payers), a reduction in the number of VOCs (9 payers), and a reduction in the number of VOCs and a decrease in use of pain medications (1 payer).
All thirteen payers included treatment continuation criteria requirements for voxelotor. These requirements included demonstrated SCD symptom control (1 payer), a decrease in blood transfusion frequency (1 payer), increased hemoglobin levels (3 payers), a reduction on SCD complications from baseline (6 payers), a reduction in SCD complications/increase in hemoglobin of more than 1.0 g/dL (2 payers).
Implications for exa-cel and lovo-cel
Our findings suggest that payers may impose similar coverage restrictions on lovo-cel and exa-cel. This speculation is supported by the fact that payers impose restrictions on gene therapies more often than for other specialty drugs and bolstered by the new therapies’ high prices.9
Payers seem likely to impose patient subgroup restrictions, and our data suggest that these restrictions may reflect the therapies’ registration studies’ inclusion criteria.
It is less clear whether plans will impose step therapy requirements. Given the annual net costs for crizanlizumab-tmca and voxelotor (estimated at over $90,000 in 2020), the large upfront payment structures of exca-cel and lovo-cel present a unique challenge.10 It will be interesting to watch whether payers require patients to first try and experience treatment failure with crizanlizumab-tmca and/or voxelotor before accessing a gene therapy.
While we did not examine Medicaid or Medicare coverage policies, our research has shown that Medicaid plans more often impose coverage restrictions for gene therapies than commercial plans, and this may prove to be the case for lovo-cel and exa-cel.9 This is important, as roughly two-thirds of SCD patients rely on Medicare and Medicaid.11
Summary
The recent approval of two SCD gene therapies has been rightly celebrated. However, payer coverage is integral to patients’ access to these much-needed treatments. We suspect that while payers will provide some degree of coverage for lovo-vel and exa-cel, they will likely restrict access to patients who closely resemble those included in the therapies’ registration studies.
More on the Specialty Drug Evidence and Coverage (SPEC) Database
The Specialty Drug Evidence and Coverage (SPEC) Database is a first-of-its-kind resource designed to enhance the transparency of commercial health plan specialty drug coverage. SPEC draws on publicly available medical and pharmacy coverage policies issued by 18 of the largest commercial health plans. Health plans in SPEC cover roughly 170 million lives, or 70% of the market. SPEC includes 429 drugs manufactured by 142 different pharmaceutical and biotech companies. We keep SPEC timely by updating its contents every four months. SPEC includes information on three principal components: (1) health plan coverage decisions, (2) the evidence health plans cite in their coverage policies, and (3) specialty drug attributes.
Access to SPEC is through the CEVR Sponsorship program. Access to the online search portal of the SPEC database is also available for individuals from academic organizations for non-commercial use of the data. Contact James Chambers (jchambers@tuftsmedicalcenter.org) for more information.
Sources:
- https://www.npr.org/sections/health-shots/2023/12/08/1217123089/fda-approves-first-gene-editing-treatments-for-human-illness
- https://newdigs.tuftsmedicalcenter.org/payingforcures/defining-disruption/therapy-distinctiveness/financial-challenges-of-cgt/
- https://www.cdc.gov/ncbddd/sicklecell/facts.html
- Johnson KM, Jiao B, Ramsey SD, Bender MA, Devine B, Basu A. Lifetime medical costs attributable to sickle cell disease among nonelderly individuals with commercial insurance. Blood Adv. 2023 Feb 14;7(3):365-374. doi: 10.1182/bloodadvances.2021006281. PMID: 35575558; PMCID: PMC9898623.
- https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm
- Beaudoin F, Thokala P, Nikitin D, Campbell J, Spackman E, McKenna A, Pearson SD, Rind DM. Gene Therapies for Sickle Cell Disease: Effectiveness and Value; Evidence Report. Institute for Clinical and Economic Review, August 21, 2023. https://icer.org/assessment/sickle-cell-disease-2023/.
- Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, Guthrie TH, Knight-Madden J, Alvarez OA, Gordeuk VR, Gualandro S, Colella MP, Smith WR, Rollins SA, Stocker JW, Rother RP. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N Engl J Med. 2017 Feb 2;376(5):429-439. doi: 10.1056/NEJMoa1611770. Epub 2016 Dec 3. PMID: 27959701; PMCID: PMC5481200.
- Vichinsky E, Hoppe CC, Ataga KI, Ware RE, Nduba V, El-Beshlawy A, Hassab H, Achebe MM, Alkindi S, Brown RC, Diuguid DL, Telfer P, Tsitsikas DA, Elghandour A, Gordeuk VR, Kanter J, Abboud MR, Lehrer-Graiwer J, Tonda M, Intondi A, Tong B, Howard J; HOPE Trial Investigators. A Phase 3 Randomized Trial of Voxelotor in Sickle Cell Disease. N Engl J Med. 2019 Aug 8;381(6):509-519. doi: 10.1056/NEJMoa1903212. Epub 2019 Jun 14. PMID: 31199090.
- Beinfeld MT, Rucker JA, Jenkins NB, de Breed LA, Chambers JD. Variation in Medicaid and commercial coverage of cell and gene therapies. Health Policy Open. 2023 Oct 13;5:100103. doi: 10.1016/j.hpopen.2023.100103. PMID: 38023441; PMCID: PMC10660088.
- Bradt P, Spackman E, Synnott PG, Chapman R, Beinfeld M, Rind DM, Pearson SD. Crizanlizumab, Voxelotor, and L-Glutamine for Sickle Cell Disease: Effectiveness and Value. Institute for Clinical and Economic Review, January 23, 2020. https://icer.org/wpcontent/uploads/2020/10/ICER_SCD_Evidence-Report_031220-FOR-PUBLICATION.pdf
- https://www.bloomberg.com/opinion/features/2023-10-15/sickle-cell-gene-therapy-will-test-how-us-pays-for-million-dollar-cures
Table 1. Coverage restrictions included in August 2023 Adekveo and Orbryta commercial coverage policies
Hemoglobin (Hb) and vaso-occlusive crises (VOC)
