By Adele Levine and James Chambers
In what would be a significant milestone for myasthenia gravis (MG) patients, the US Food and Drug Administration (FDA) is expected to approve efgartigimod later this year. Efgartigimod would be only the second biologic therapy available for MG. If and when FDA approves efgartigimod, will commercial health plan coverage facilitate patient access?
What is myasthenia gravis?
Myasthenia gravis is a debilitating autoimmune neuromuscular disorder characterized by muscle weakness and fatigue.1 Most patients with MG progress to generalized MG (gMG), with the disease affecting multiple muscle groups, such as the face, neck, and limbs.2 gMG is a rare disease that affects 10-20 individuals per 100,000, although research suggests it is underdiagnosed.3
Conventional treatments include corticosteroids and immunosuppressive drugs, either alone or in combination. However, gMG patients often cannot tolerate these treatments or find them to be ineffective. FDA approved eculizumab (SolirisTM), the only biologic treatment currently available for gMG for “adult patients who are anti-acetylcholine receptor antibody positive.”4
In its recent assessment of MG treatments, the Institute for Clinical and Economic Review (ICER) reported that both eculizumab and efgartigimod appear to significantly improve functioning and quality of life for MG patients, although ICER concluded the evidence was insufficient to compare the two treatments.5
How might health plans cover efgartigimod?
Coverage polices reflect a complex set of inputs and health plans serve different populations. Plans and drug manufacturers rarely disclose pricing or rebate agreements, which undoubtedly influence coverage policy formulation. Still, we can gain insight into how health plans may cover efgartigimod by examining how they have covered eculizumab, another treatment for gMG that is similar to efgartigimod. We focus on two utilization management tools and identify measures that may limit access to the drug by patients indicated for treatment by the FDA:
- Clinical requirements, which specify the clinical criteria patients must satisfy, such as symptom severity or duration; and
- Step-therapy protocols, which require patients to first try one or more alternative treatments and experience treatment failure before they can receive eculizumab.
Our data come from the Tufts-CEVR Specialty Drug Evidence and Coverage (SPEC) Database, which contains detailed information on how 17 of the largest US commercial health plans cover specialty drugs and products.
Clinical coverage requirements
All 17 commercial health plans imposed restrictions in their coverage policies that limit access to eculizumab among patients that FDA indicated for treatment. Sixteen plans required patients to have a Myasthenia Gravis Foundation of America (MGFA) clinical classification between II and IV, which excludes gMG patients with the least severe form of the disease (ocular weakness only) and the most severe form (requiring intubation).6
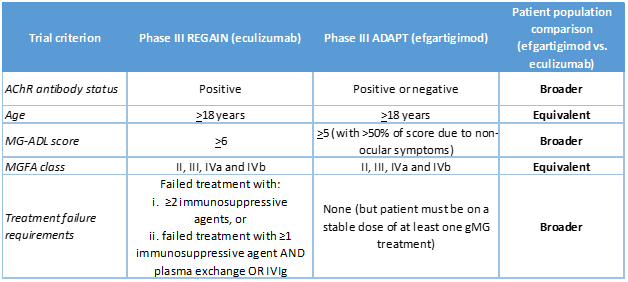
Fifteen plans required a score of at least 6 on the 8-item MG-Activities of Daily Living (MG-ADL) scale, meaning patients must have at least some difficulty with or fatigue when engaging in daily activities such as talking, chewing, and getting up from a chair. These clinical requirements are consistent with the inclusion criteria for the product’s phase III trial (REGAIN) (NCT01997229) (Table 1), but are not included in FDA’s indication criteria.
One health plan did not impose MGFA clinical classification or MG-ADL-related coverage requirements but required patients to experience at least one gMG-related symptom, such as impaired swallowing, extremity weakness, disabling fatigue, or shortness of breath.
Step therapy requirements for gMG
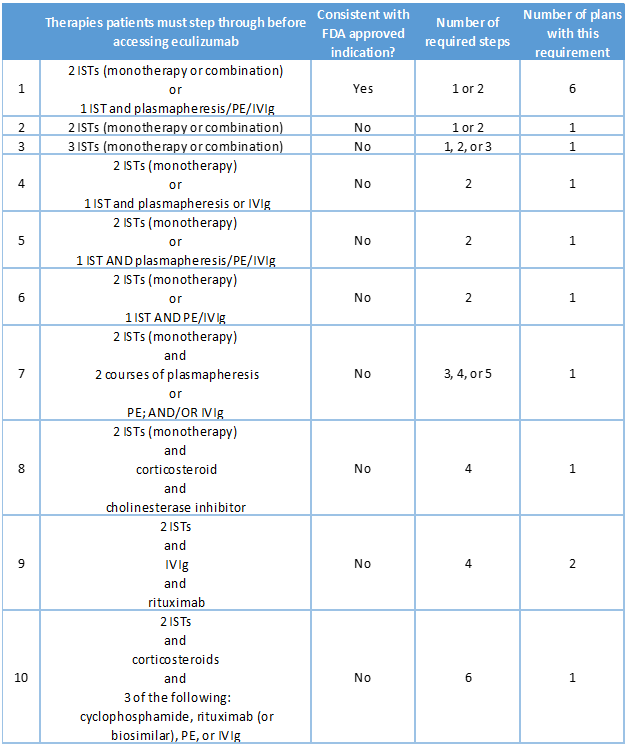
Coverage policies for eculizumab issued by sixteen plans imposed step therapy protocols. For six plans, protocols were consistent with the eculizumab’s clinical trial inclusion criteria4:
- Failed treatment over 1 year or more with 2 or more immunosuppressive therapies (ISTs) either in combination or as monotherapy, or failed at least 1 IST and required chronic plasmapheresis or plasma exchange (PE) or intravenous immunoglobulin (IVIg)
Step therapy requirements imposed by the other 11 plans step varied widely (Table 2).
What are the implications for efgartigimod?
Plans have imposed onerous coverage restrictions on eculizumab, likely because of the treatment’s high price and unfavorable cost-effectiveness. ICER estimated that eculizumab’s price would need to decline by as much as 98 percent in order to represent acceptable value.5 If efgartigimod’s pricing strategy is similar, health plans might follow suit with comparable coverage restrictions.
Clinical criteria
As most plans did in the case of eculizumab, we expect coverage policies for efgartigimod to impose clinical criteria that likewise correspond to clinical trial inclusion criteria. That is, plans may require patients to have an MG-ADL score of at least 5 and an MGFA class designation of II, III, or IV (Table 1).
Step therapy
Although clinical trials for efgartigimod imposed less-stringent prior therapy inclusion requirements on subjects than the trials for eculizumab imposed, we expect coverage policy step therapy requirements for efgartigimod to be largely consistent with the corresponding coverage requirements for eculizumab. Requiring patients to try more conservative treatments can reduce costs because those treatments are much less expensive than efgartimod. Step requirements will likely vary across plans, but plans may require patients to try some combination of immunosuppressive therapy, plasmapheresis, plasma exchange, or intravenous immunoglobulin, all of which are conservative treatments.
Finally, it is likely that coverage requirements for efgartigimod will vary across plans, just as they do for eculizumab. Those differences can be problematic for patients’ access, as it means drug coverage depends on which health insurance plan they happen to belong to.
One last wrinkle is how health plans will manage coverage of efgartigimod and eculizumab. While plans often prioritize coverage of some targeted therapies over others for chronic diseases (e.g., rheumatoid arthritis treatments), plans less often do so for treatments for rare diseases. Whether plans will require patients to first try and fail eculizumab before being eligible for efgartigimod (or vice-versa) is unclear, as that decision will depend on relative pricing and plans’ preparedness to limit their enrollees’ access to available treatments.
More on the Specialty Drug Evidence and Coverage (SPEC) Database
The Specialty Drug Evidence and Coverage (SPEC) Database is a first-of-its-kind resource designed to enhance the transparency of commercial health plan specialty drug coverage. SPEC draws on publicly available medical and pharmacy coverage policies issued by 17 of the largest commercial health plans. Health plans in SPEC cover roughly 164 million lives, or 63% of the market. SPEC includes 367 drugs manufactured by 101 different pharmaceutical and biotech companies. We keep SPEC timely by updating its contents every four months. SPEC includes information on three principal components: (1) health plan coverage decisions, (2) the evidence health plans cite in their coverage policies, and (3) specialty drug attributes.
Access to SPEC is through the CEVR Sponsorship program. Access to the online search portal of the SPEC database is also available for individuals from academic organizations for non-commercial use of the data. Contact James Chambers (jchambers@tuftsmedicalcenter.org) for more information.
Tables
Table 1. Comparison of eculizumab and efgartigimod phase III trial populations
Table 2. Step therapies included in August 2021 eculizumab coverage policies
1IST = Immunosuppressive therapy; 2PE = Plasma exchange; 3IVIg = Intravenous immunoglobulin
References
1. Gilhus NE. Myasthenia Gravis. New England Journal of Medicine 2016;375:2570-81.
2. Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine 2008;37:141-9.
3. Hehir MK, Silvestri NJ. Generalized myasthenia gravis: classification, clinical presentation, natural history, and epidemiology. Neurologic clinics 2018;36:253-60.
4. SOLIRIS® (eculizumab) injection, for intravenous use 2020. at www.accessdata.fda.gov/drugsatfda_docs/label/2020/125166s434lbl.pdf
5. Tice JA ND, Campbell J, Moradi A, Rind DM, Pearson SD, Agboola F. Eculizumab and Efgartigimod for the Treatment of Myasthenia Gravis: Effectiveness and Value; Final Report.: Institute for Clinical and Economic Review; September 10, 2021.
6. Jaretzki A, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Neurology 2000;55:16-23.