By James Chambers, PhD, MPharm, Associate Professor and Nola Jenkins, BA, Research Assistant
Key points
- The largest US commercial health plans vary in how they cover dupilumab for moderate-to-severe atopic dermatitis.
- Plans’ clinical coverage criteria have become more consistent over time.
- Fewer plans are now requiring that a dermatologist prescribe dupilumab compared to 2017.
- Plans step therapy requirements vary widely, with the number of “steps” required before patients can access dupilumab ranging from one to five.
In this blog we examine how commercial health plan coverage of dupilumab (Dupixent®) for the treatment of moderate-to-severe atopic dermatitis has evolved since the product’s FDA approval in 2017. FDA also approved dupilumab for asthma (2018) and chronic rhinosinusitis with nasal polyposis (2019). Atopic dermatitis affects roughly 15% of children and 10% of adults in the US.1,2 The majority of children suffer from a mild form of the disease; moderate-to-severe atopic dermatitis is more common in adults.3,4 Dupilumab is widely regarded as a safe and effective treatment.5 Dupilumab is currently the only available biologic therapy available for moderate-to-severe atopic dermatitis, although it will soon face competition.6
The Tufts-CEVR Specialty Drug Evidence and Coverage (SPEC) Database, contains detailed information from 2017-2021 on how plans cover specialty drugs and products. SPEC quantifies payer behavior by translating publicly available specialty drug coverage policies into structured, analysis-ready data.
We used SPEC to scrutinize how the included US commercial health plans (representing roughly 150 million lives; 60% of the commercial market) covered dupilumab for moderate-to-severe atopic dermatitis from 2017 through 2021. We focus on three broad categories of plan imposed utilization management criteria:
- Clinical requirements – Plan requires a patient to meet specific clinical criteria, e.g., to have experienced symptoms of particular severity or duration, before they can access dupilumab.
- Prescriber requirements – Plan requires dupilumab to be prescribed by a dermatologist, or in consultation with a dermatologist.
- Step-therapy protocols – Plan requires a patient to try one or more alternative treatments, and experience treatment failure, before they can access dupilumab.
Clinical criteria
When health plans imposed clinical criteria in their coverage policies they tended to require one or more of the following:
- ≥10% body surface area (BSA) affected (or involvement of sensitive areas of the body)
- Particular symptoms to be present, e.g., erythema, edema, lichenification, crusting and oozing
- The patient’s symptoms have been present for ≥3 years
- Investigator’s Global Assessment (IGA) score of 3 or 4 (the overall assessment of AD lesions on a severity scale of 0 to 4)
As important context, the requirement for a minimum BSA involvement of ≥10% and an IGA score ≥3 were among dupilumab’s FDA registration studies’ inclusion criteria; the ≥3 year symptom duration and requirement that certain symptoms be present were not.
Health plans’ clinical requirements have changed over time (Figure 1). Notably, the percentage of plans requiring eligible patients to have ≥10% BSA affected (or have sensitive body area involvement) increased from 30% in 2017 to 57% in 2021. Conversely, the percentage of plans requiring that a patient’s symptoms have been present for ≥3 years before being eligible for dupilumab decreased from 30% in 2017 to 0% in 2021. One plan required a patient have an IGA score ≥3 in their 2017-2019 coverage policies, but removed the requirement in 2020. One other plan required that the patient be suffering from particular symptoms, e.g., erythema, edema, lichenification, crusting and oozing, at each of the five time points.
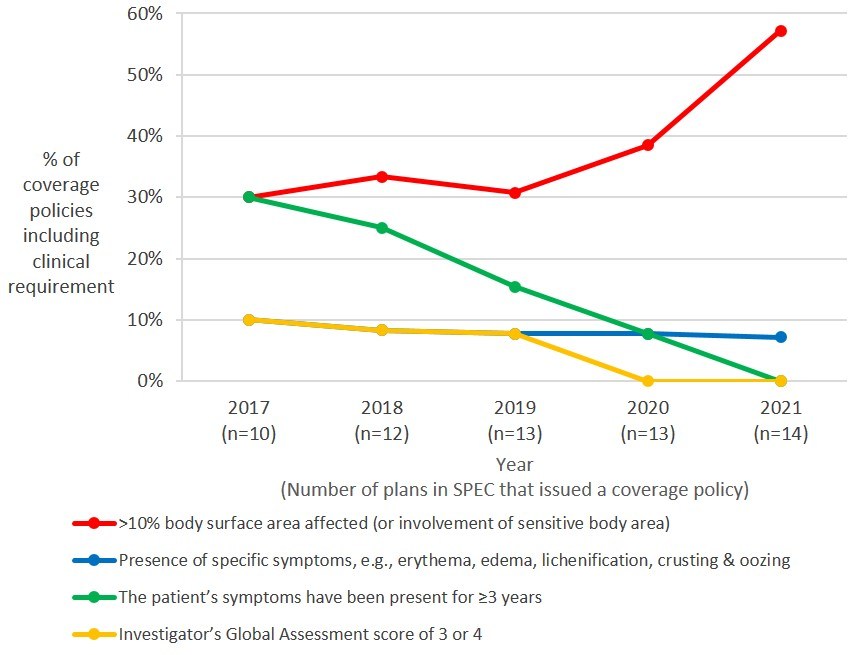
Exhibit 1. Clinical criteria embedded in health plans’ coverage policies (2017-2021)
Note: The FDA initially approved dupilumab only for adults with atopic dermatitis. The FDA has twice adjusted the age of eligibility in its approval: first to ≥12 years of age (March, 2019), second to ≥6 years of age (May, 2020). On occasion, there was a delay in a plan adjusting its coverage criteria to reflect these label changes. We do not report these differences in this blog.
Prescriber requirements
Health plans often include a requirement in their specialty drug coverage policies that either a specialist physician prescribes the drug, or that the prescriber consults with a specialist physician before doing so. While the percentage of dupilumab coverage policies including prescriber requirements remained relatively steady over the five-year period, the requirements have become less stringent. In 2017, 40% of plans’ required that a dermatologist prescribe dupilumab, compared to only 21% of plans in 2021 (Exhibit 2).
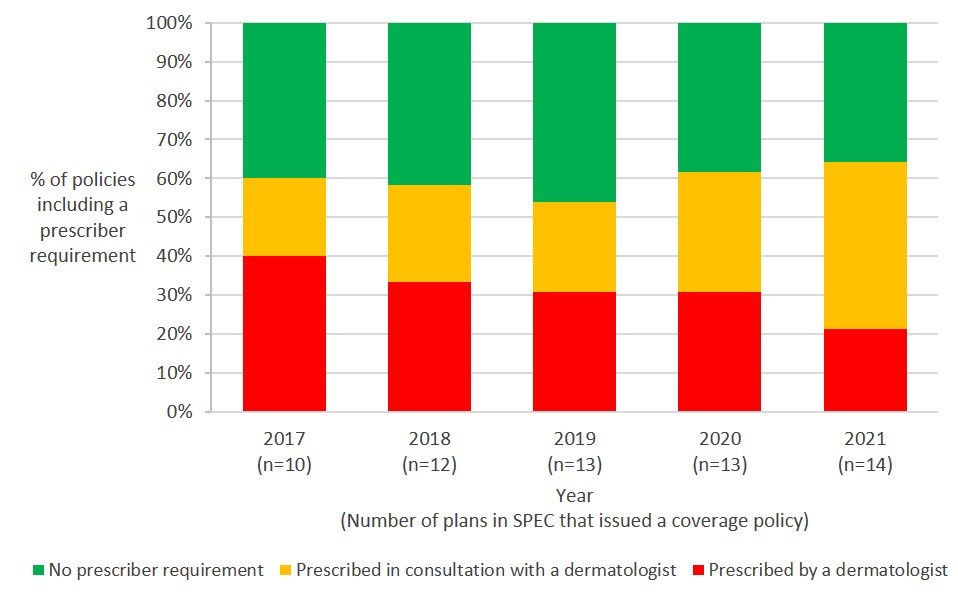
Exhibit 2. Health plans’ prescriber requirements for dupilumab (2017-2021)
Step therapy requirements
The FDA approved dupilumab for patients whose disease is not adequately controlled with topical prescription therapies; however, health plans often imposed additional step therapy requirements in their coverage policies. In aggregate terms, plans’ step therapy requirements became somewhat less stringent over time, with the average number of required steps decreasing from 3.3 in 2017 to 2.9 in 2021. However, there was wide variation across the step therapy requirements.
Exhibit 3 includes the six different step therapy protocols the included health plans included in their August 2021 coverage policies. Three plans required patients to first fail a single topical treatment (topical steroid or calcineurin inhibitor), while five plans required patients to fail a topical steroid and a calcineurin inhibitor. One plan required patients to fail four prior treatments: two topical steroids, one calcineurin inhibitor, and one systemic agent. One plan required patients to fail five prior treatments: two topical steroids, one calcineurin inhibitor, phototherapy, and one systemic agent.
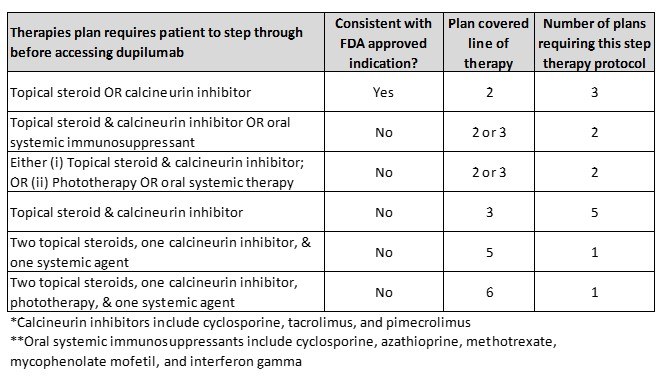
Exhibit 3. Required step therapy included in August 2021 coverage policies
Notes: Calcineurin inhibitors include cyclosporine, tacrolimus, and pimecrolimus; Systemic agents include corticosteroids or immunosuppressant therapy (e.g., cyclosporine, azathioprine, methotrexate, mycophenolate mofetil, and interferon gamma); Phototherapy includes PUVA (a combination of psoralen and long-wave ultraviolet radiation) and UVB (narrowband ultraviolet B light). *One plan had different step requirements for patients with moderate disease than it had for patients with severe disease. This plan required patients with moderate disease to step through a topical steroid, a calcineurin inhibitor and crisaborole (Eucrisa®); the same plan required patients with severe disease to step through a topical steroid and a calcineurin inhibitor.
Discussion
The largest US commercial health plans have relaxed their coverage criteria for dupilumab for the treatment of moderate-to-severe atopic dermatitis since its FDA approval in 2017. From 2017 to 2021 plans removed certain clinical requirements, less often required a dermatologist to prescribe the drug, and required patients to first try and fail fewer prior treatments before accessing dupilumab. The increasing number of plans requiring that patients have >10% BSA affected (or have sensitive body area involvement) may reflect a growing consensus on the diagnostic criteria for moderate-to-severe atopic dermatitis.
There are a number of potential explanations for why plans would relax their coverage criteria. Dupilumab was the first biologic approved for moderate-to-severe atopic dermatitis and it is perhaps unsurprising that plans initially showed a degree of caution in their coverage policies. Health care providers’ growing experience with dupilumab, a maturing evidence base supporting the product, and its favorable safety, effectiveness, and cost-effectiveness profile7 may have led plans to relax their coverage requirements. Our findings are consistent with prior research that found that plans tend to cover specialty drugs more generously the longer they are on the market.8,9
Health plans’ coverage policies have varied widely. Most notably, some plans’ step therapy protocols were much more stringent than other plans’ protocols. This variation is important, as it means that patients’ access to dupilumab may be affected by their insurance coverage. Differences among plan coverage policies may also mean that health care providers must tailor their treatment not only to the patient’s clinical presentation, but also to the patient’s insurance coverage.
An important limitation of this analysis is that we do not account for patient cost-sharing, which presents a significant hurdle for many patients. While this analysis included 14 of the largest commercial health plans, our findings may not generalize to other private and public health care payers.
We will continue to monitor how the health plans included in SPEC cover dupilumab, including how plans respond to the introduction of other biologic therapies for atopic dermatitis, and the extent to which the introduction of these therapies affects how plans cover dupilumab.
More on the Specialty Drug Evidence and Coverage (SPEC) Database
The Specialty Drug Evidence and Coverage (SPEC) Database is a first-of-its-kind resource designed to enhance the transparency of commercial health plan specialty drug coverage. SPEC draws on publicly available medical and pharmacy coverage policies issued by 17 of the largest commercial health plans. Health plans in SPEC cover roughly 164 million lives, or 63% of the market. SPEC includes 367 drugs manufactured by 101 different pharmaceutical and biotech companies. We keep SPEC timely by updating its contents every four months. SPEC includes information on three principal components: (1) health plan coverage decisions, (2) the evidence health plans cite in their coverage policies, and (3) specialty drug attributes.
Access to SPEC is through the CEVR Sponsorship program. Access to the online search portal of the SPEC database is also available for individuals from academic organizations for non-commercial use of the data. Contact James Chambers (jchambers@tuftsmedicalcenter.org) for more information.
References
1. McKenzie C SJ. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123(2):173-178.e171.
2. Silverberg JI HJ. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132(5):1132-1138.
3. Silverberg JI SE. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25(3):107-114
4. Ballardini N KI, Söderhäll C, Lilja G, Wickman M, Wahlgren CF. Eczema severity in preadolescent children and its relation to sex, filaggrin mutations, asthma, rhinitis, aggravating factors and topical treatment: a report from the BAMSE birth cohort. Br J Dermatol. 2013;168(3):588-594
5. Frazier W, Bhardwaj N. Atopic Dermatitis: Diagnosis and Treatment. Am Fam Physician. 2020 May 15;101(10):590-598
6. National Eczema Association. JAK Inhibitors Are Coming and They Are the Biggest Eczema Development in Years. Available: https://nationaleczema.org/jak-inhibitors-research/
7. Institute for Clinical and Economic Review. Dupilumab and Crisaborole for Atopic Dermatitis: Effectiveness and Value Final Evidence Report and Meeting Summary June 8, 2017. Available: https://icer.org/wp-content/uploads/2020/10/MWCEPAC_ATOPIC_FINAL_EVIDENCE_REPORT_060717.pdf
8. Chambers JD, Panzer AD, Kim DD, Margaretos NM, Neumann PJ. Variation in US private health plans' coverage of orphan drugs. Am J Manag Care. 2019 Oct;25(10):508-512
9. Chambers JD, Kim DD, Pope EF, Graff JS, Wilkinson CL, Neumann PJ. Specialty Drug Coverage Varies Across Commercial Health Plans In The US. Health Aff (Millwood). 2018 Jul;37(7):1041-1047
